The way our genetic material works is that some genes are transcribed, that is, that their information is read and used to create a protein with a particular function, and that other genes, however, are not read, are not transcribed despite being encoded in our DNA.
Whether a cell becomes a neuron, a skin cell, or a muscle cell is determined by which genes are being read in each case. DNA methylation, in fact, regulates which genes are expressed and which are not. Simply put, methylated regions silence genes, and nonmethylated regions express genes. As previously mentioned, as we age, our DNA methylation levels decrease, and we have less control over our DNA..
Dr. Alfonso Galán González – Neolife Medical Team
Unlike the permanent mutations in our genes, which we cannot change, we do have the ability to change this epigenome.
In this blog post, we’ll present one of the famous 9 “Hallmarks of Aging”, or factors that determine aging, which are, in fact, reversible. We’re talking about epigenetic alterations.
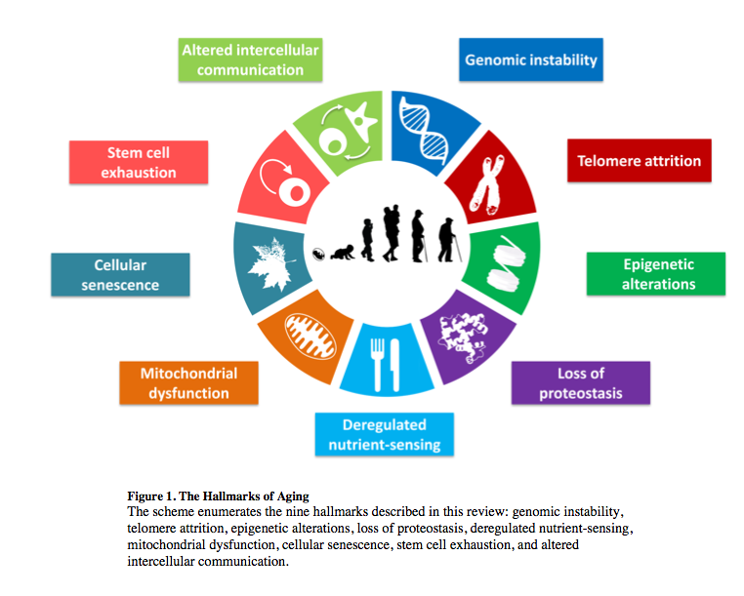
Epigenetics is not the easiest thing to explain, but we’ll give it a try. If we consider the word “epigenetics”, we may see it means something that is “epi” or “over” or “on top of” our genes. Something that is not written in our genes, in our DNA, but that impacts how our genes are going to work. The way our genetic material works is that some genes are transcribed, that is, that their information is read and used to create a protein with a particular function, and that other genes, however, are not read, are not transcribed despite being encoded in our DNA. Whether a cell becomes a neuron, a skin cell, or a muscle cell is determined by which genes are being read in each case, even though all these cells have the same genetic material. So, our genome is the complete list of all the genes that we can potentially read or not read, activate or not, turn ON or OFF, and what determines this? Our epigenome.
Our epigenome are a series of markers added to our DNA without modifying its sequence, but which determine exactly that, what should be expressed and what should not.
All our cells have 23 chromosomes. Chromosomes are a tangle of chromatin, formed by nucleosomes, which look like the beads of a necklace, which are in turn made up of DNA coiled around proteins called histones. This is how structural support is given to the chromosomes and they fit in the nucleus of the cell.
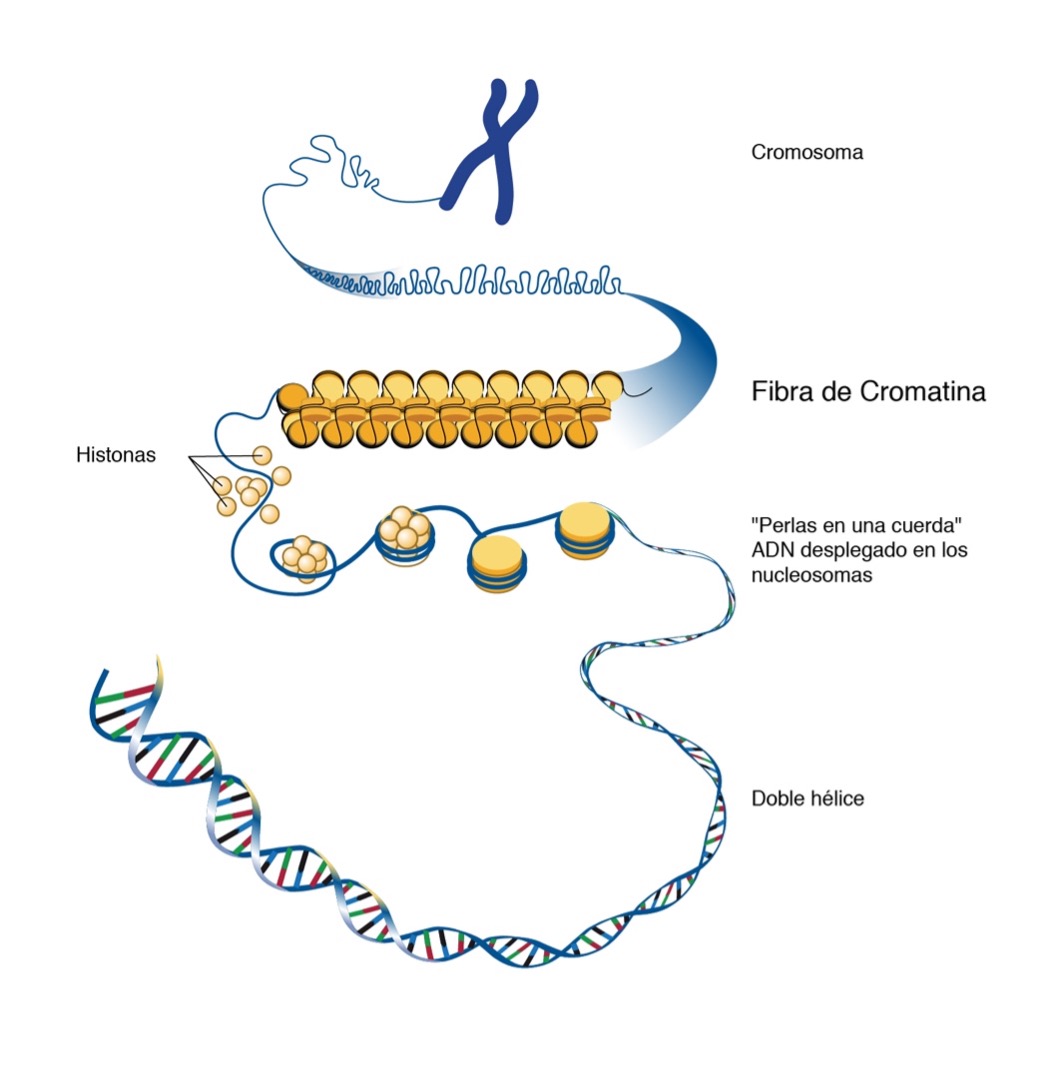
Epigenetic alterations consist of changes in this chromatin and may be changes in both the histones and chromatin structure, as well as the addition of methyl groups to the DNA. These alterations may be inherited by daughter cells and therefore perpetuated.
Let’s delve deeper into the issue of DNA methylation (1).
DNA methylation, in fact, regulates which genes are expressed and which are not. Methylation patterns change over time. Simply put, though it’s actually a bit more complicated, methylated regions silence genes, and nonmethylated regions express genes. As previously mentioned, as we age, our DNA methylation levels decrease, and we have less control over our DNA (3).
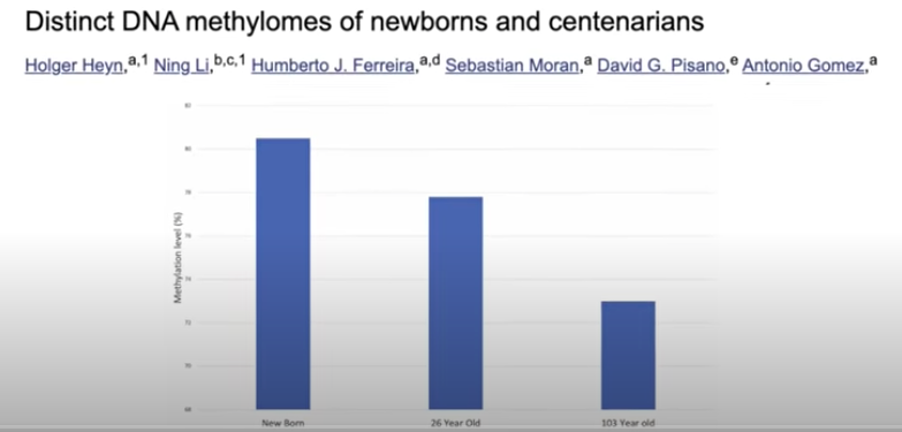
This influences the function of the cells, harming them and predisposing them to the appearance of a pathology. Yes, indeed, we’re talking about the often mentioned diseases associated with aging. We now know that these epigenetic alterations, namely this change in DNA methylation patterns predispose to cancer, neurodegenerative diseases, obesity, diabetes, insulin resistance, inflammation, cardiovascular disease, immune disorders, etc. (2). These epigenetic alterations accumulate as we age, and we can measure them. Multiple “epigenetic clocks” such as Horvath’s and more complete current ones have been designed, which are used to measure just how methylated a series of genes are, giving us an idea of our biological age as opposed to our chronological age. Tests are now available that measure the methylation of millions of sites in the genome when initially only a few hundred (4) were available.
Unlike the permanent mutations in our genes, which we cannot change, we can modify this epigenome, and there are multiple studies that show how we can make these clocks start working backwards and rejuvenate our biological age, based on what they measure.
How can we do this?
Well, you’ve probably heard all this before: exercise, a balanced diet, and supplementation.
A systematic review of the scientific literature published in January this year shows us that strength training in humans induces epigenetic alterations in pathways associated with energy metabolism and insulin sensitivity, contributing to skeletal muscle health. Aerobic exercise also causes modifications in biomarkers associated with metabolic alterations through changes in specific DNA methylation and mRNA expression. It seems that the best strategy is a combination of both types of exercise (6).
Calorie restriction, which we’ve already discussed in previous blog posts, prevents a decrease in DNA methylation levels, even in different organs (5).
Calorie restriction mimetics, like metformin or resveratrol have also demonstrated these benefits.
Smoking and psychological trauma lead to changes in methylation patterns.
The availability of methyl group donor molecules influences our methylation levels. Betaine (trimethyl glycine –TMG-) is a safe supplement that helps us with this task (7). Additionally, it lowers homocysteine levels; you may see why this is important here.
In short, a decrease in DNA methylation leads to pathology and aging. This process may be reversed through exercise, a proper diet, and supplements like TMG.
We cannot emphasize enough how important it is to exercise and maintain a good diet. This is just more scientific proof of their incredible benefits.
BIBLIOGRAPHY
(1) Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013 Jan;38(1):23-38. doi: 10.1038/npp.2012.112. Epub 2012 Jul 11. PMID: 22781841; PMCID: PMC3521964.
(2) Sallustio F, Gesualdo L, Gallone A. New findings showing how DNA methylation influences diseases. World J Biol Chem. 2019 Jan 7;10(1):1-6. doi: 10.4331/wjbc.v10.i1.1. PMID: 30622680; PMCID: PMC6314879.
(3) Heyn H, Li N, Ferreira HJ, Moran S, Pisano DG, Gomez A, Diez J, Sanchez-Mut JV, Setien F, Carmona FJ, Puca AA, Sayols S, Pujana MA, Serra-Musach J, Iglesias-Platas I, Formiga F, Fernandez AF, Fraga MF, Heath SC, Valencia A, Gut IG, Wang J, Esteller M. Distinct DNA methylomes of newborns and centenarians. Proc Natl Acad Sci U S A. 2012 Jun 26;109(26):10522-7. doi: 10.1073/pnas.1120658109. Epub 2012 Jun 11. PMID: 22689993; PMCID: PMC3387108.
(4) Bell CG, Lowe R, Adams PD, Baccarelli AA, Beck S, Bell JT, Christensen BC, Gladyshev VN, Heijmans BT, Horvath S, Ideker T, Issa JJ, Kelsey KT, Marioni RE, Reik W, Relton CL, Schalkwyk LC, Teschendorff AE, Wagner W, Zhang K, Rakyan VK. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 2019 Nov 25;20(1):249. doi: 10.1186/s13059-019-1824-y. PMID: 31767039; PMCID: PMC6876109.
(5) Gensous N, Franceschi C, Santoro A, Milazzo M, Garagnani P, Bacalini MG. The Impact of Caloric Restriction on the Epigenetic Signatures of Aging. Int J Mol Sci. 2019 Apr 24;20(8):2022. doi: 10.3390/ijms20082022. PMID: 31022953; PMCID: PMC6515465.
(6) Barrón-Cabrera E, Ramos-Lopez O, González-Becerra K, Riezu-Boj JI, Milagro FI, Martínez-López E, Martínez JA. Epigenetic Modifications as Outcomes of Exercise Interventions Related to Specific Metabolic Alterations: A Systematic Review.
Lifestyle Genom. 2019;12(1-6):25-44. doi: 10.1159/000503289. Epub 2019 Sep 23. PMID: 31546245; PMCID: PMC6921698.
(7) Zhao G, He F, Wu C, Li P, Li N, Deng J, Zhu G, Ren W, Peng Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front Immunol. 2018 May 24;9:1070. doi: 10.3389/fimmu.2018.01070. PMID: 29881379; PMCID: PMC5976740.

